Classification of candidates for clinical trials with Cuban drug against Alzheimer advances in Havana
The classification consultations of the patients who will participate in the phase III clinical trial with the Cuban neuroprotective drug against Alzheimer's NeuralCIM have been held since Tuesday, July 5, in eight health centers in Havana.
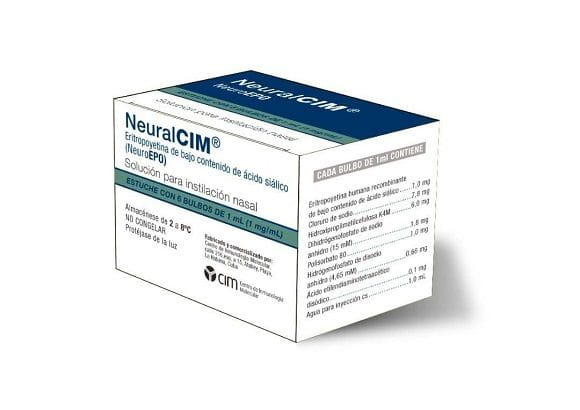
The positive results of the phase II/III clinical trial in Cuba with the product or NeuralCIM (commercial name of the NeuroEPO molecule), created by the Molecular Immunology Center (CIM), allowed the Center for the State Control of Medicines, Equipment and Medical Devices (CECMED) will grant on March 8, 2022 a Conditional Sanitary Registry, valid for three years, to use this national drug in patients with moderate and mild Alzheimer's, with the purpose of expanding and consolidating the results obtained in this phase of development. clinical.
Then Dr. C. Teresita Rodríguez, project manager and CIM specialist, in statements to the press, highlighted that after the first clinical investigations carried out in Cuba, the nasal formulation showed a low level of adverse effects, anti-inflammatory action, while it increases blood irrigation, promotes the formation of new blood vessels and is an antioxidant.
For his part, Doctor of Science Kalet León Monzón, first deputy director of the Center for Molecular Immunology (CIM), in recent statements to Granma, explained that the previous research being carried out in the capital, although it does not mean the full start of the clinical trial, constitutes an important step to advance in the correct diagnosis of patients and in the identification of the population that will be included.
This media reports that the promoter of the CIM Alzheimer's clinical trial, Doctor of Science Leslie Pérez Ruiz, reported that the country currently has a database of more than 400 patients, of which 371 are from the capital.
She also commented that to carry out the trial, 310 of the caregivers have been located, who can call and are assigned a shift for the consultation, in which they will identify if the patient has a mild / moderate Alzheimer's pattern.
According to Pérez Ruiz, phase III of the study is likely to start next September or October, and it seeks to evaluate the efficacy and safety of this nasally administered drug.
She announced that the trial will compare NeuralCIM with Donepezil, a drug approved since the 1990s by the United States Drug Regulatory Agency.
Regarding the particularities of the clinical investigations, he said that in Havana, in addition to the clinical determination, and for the first time in Cuba, through the determination of beta-amyloid proteins in the cerebrospinal fluid, whose accumulation in the brain is identified as a precursor to Alzheimer's, the patient will be given a molecular diagnosis of this disease.
She added that a phase IV trial will be carried out in other provinces of the country, specifically in patients with a mild/moderate disease phenotype, which includes the amnesic variant, whose diagnosis will only be clinical.
Dr. Pérez Ruiz added that the medication will be applied by the caregiver at home, who will be trained in the Health institutions for the correct administration of the NeuralCIM.
How to participate in the trial
A note published by BioCubafarma on the consultations for the classification of patients for the phase III clinical trial in mild and moderate Alzheimer's, specifies that everyone who wishes to participate must first request an appointment through the established contacts.
It also specifies that the current stage is patient classification, so only those who meet the selection requirements will be included in the clinical trial.
The start of the trial is scheduled for September, once it is approved by the Center for the State Control of Medicines, Equipment and Medical Devices (CECMED).
In Havana, caregivers or family members interested in requesting shifts for consultations should go to the Institute of Neurology and Neurosurgery, the International Center for Neurological Restoration, the Medical Surgical Research Center, the Cristóbal Labra polyclinic, and the Enrique Cabrera and Salvador Allende hospitals. and Ameijeiras Brothers.
Graphic taken from Granma
NeuralCIM, Alzheimer and incidence in Cuba
The results of the phase II/III clinical trial with NeuroEPO, a variant of recombinant human erythropoietin and formulated for nasal administration, in patients with moderate and mild Alzheimer's showed that there is a halt in the progression of the disease and that it improves aspects related to cognitive sphere.
The NeuroEPO molecule, an erythropoietin with a low sialic acid content very similar to the one that naturally originates in the brain, has been shown to have beneficial effects in patients with mild and moderate Alzheimer's.
The NeuralCIM seeks to restore the imbalance caused by neurodegenerative conditions in the brain and stabilize its physiological functioning.
Read Summary of NeuralCIM Features
In the treatment of Alzheimer-type dementias in mild and moderate stages with this innovative drug, the CIM experts report that there is an arrest in the progression of the disease, aspects related to the cognitive sphere improve, and there are few adverse events.

This was confirmed by the CIM specialist, Dr.C Teresita Rodríguez, who in statements to the Scientific Observatory space recounted the benefits appreciated by her mother after being treated with Neuralcim:
“I noticed that mom learned to count again and started to communicate with the caregiver.” So I said to myself: this product has an extraordinary capacity to be able to have very beneficial results”.
On video: Testimony of Dr.C Teresita Rodríguez
The drug has shown potential and its future use in the treatment of neurodegenerative diseases such as Parkinson's, Ataxias and some brain conditions is also being evaluated.
Official data from the National Statistics Office of Cuba indicates that in 2020, 21.3 percent of the Cuban population is 60 years old or older, and it is estimated that by 2050 it will be 34.9 percent. These values determine that the Caribbean nation is currently the oldest country in the region, and that in 2050, it will be one of the countries with the highest rate of demographic aging on the planet.
Official data link of the National Statistics Office of Cuba http://www.onei.gob.cu/sites/default/files/000_aging_de_la_poblacion.2020.pdf
According to this report, Havana is one of the seven provinces with the highest values of aging, with 1,546 elderly people for every 1,000 children and young people, respectively.
On the other hand, according to official statistics cited by Cubadebate, about 170,000 people suffer from dementia in Cuba, which means 10% of those over 65 years of age, and 1.3% of the general population. The prevalence of dementia syndrome in our country is estimated at 10.2 per 100 people aged 65 and over, with Alzheimer's disease being its most frequent cause.
Related information
Five questions about NeuroEPO, the Cuban drug to slow Alzheimer's